CPOS Seminar: Chemical Recycling of Polyethylene by Tandem Catalytic Conversion to Propylene

Speaker: Garrett Strong, Graduate Student Researcher, Chemical Engineering, UCSB, Scott Lab
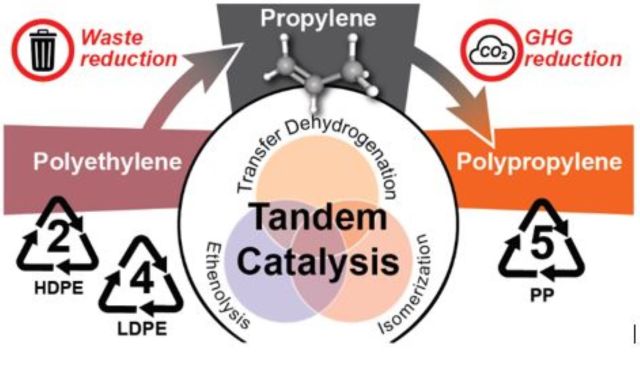
Although polyethylene (PE) and polypropylene (PP) are by far the world’s largest volume plastics, only a tiny fraction of these energy-rich polyolefins is currently recycled. Depolymerization of PE to its constituent monomer, ethylene, is highly endothermic and conventionally accessible only through unselective, high temperature pyrolysis. Instead, a tandem catalysis strategy serves to convert PE to propylene, the commodity monomer used to make PP. The approach combines rapid olefin metathesis with rate-limiting isomerization. Mono-unsaturated PE is progressively disassembled at modest temperatures via many consecutive ethenolysis events, resulting ultimately in propylene. Fully saturated PE can be converted to unsaturated PE starting with a single transfer dehydrogenation to ethylene, which produces a small amount of ethane (one equiv. per dehydrogenation event). These principles are demonstrated using both homogeneous and heterogeneous catalysts. While selectivity under batch conditions is limited at high conversion by formation of an equilibrium mixture of olefins, high selectivity to propylene (greater than 95 %) is possible in a semi-continuous process due to the continuous removal of propylene from the reaction mixture.